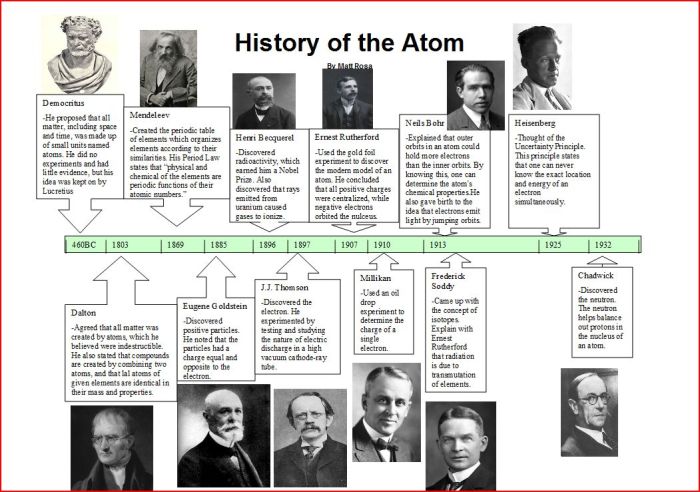
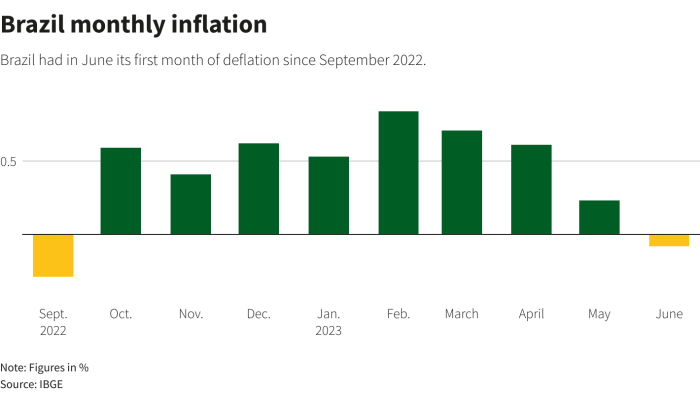Embark on a captivating journey through the history of the atom timeline project, a chronicle that traces the evolution of scientific thought regarding the nature of matter. From the ancient Greeks to modern quantum mechanics, this project delves into the groundbreaking discoveries and contributions of prominent scientists who have shaped our understanding of the atom.
The narrative unfolds chronologically, highlighting key milestones such as Dalton’s atomic theory, Thomson’s electron discovery, Rutherford’s nuclear model, and Bohr’s atomic model. Along the way, we explore the development of atomic models, from the plum pudding model to the quantum mechanical model, examining their key features and limitations.
Introduction
Understanding the history of the atom is crucial for comprehending the evolution of scientific thought and the development of our current understanding of the fundamental building blocks of matter.The concept of the atom has undergone a remarkable transformation over centuries, from the indivisible particles proposed by ancient Greek philosophers to the complex subatomic world revealed by modern physics.
This journey has not only shaped our understanding of the physical world but has also laid the foundation for numerous technological advancements.
Early Conceptions of Matter
Early civilizations, such as the Greeks, believed matter to be continuous and infinitely divisible. However, in the 5th century BC, Democritus proposed the existence of indivisible particles called atoms, a concept later adopted by Roman philosopher Lucretius. These early atomic theories lacked experimental evidence and were largely speculative.
Dalton’s Atomic Theory
In the early 19th century, John Dalton’s groundbreaking work provided the first scientific basis for the atomic theory. Dalton’s experiments with gases led him to propose that matter is composed of indivisible atoms of different weights and that chemical reactions involve the rearrangement of these atoms.
Discovery of the Electron
The discovery of the electron by J.J. Thomson in 1897 marked a significant turning point in atomic physics. Thomson’s experiments with cathode rays demonstrated the existence of negatively charged particles within atoms, challenging the prevailing view of atoms as indivisible entities.
Rutherford’s Nuclear Model
In 1911, Ernest Rutherford’s gold foil experiment revealed the existence of a tiny, dense nucleus at the center of the atom. This discovery overturned the plum pudding model of the atom, which had proposed a uniform distribution of positive and negative charges.
Bohr’s Model and Quantum Mechanics
Niels Bohr’s 1913 model of the atom introduced the concept of energy levels and explained the emission and absorption of light by atoms. Bohr’s model was later superseded by quantum mechanics, which provided a more comprehensive and accurate description of atomic structure and behavior.
Timeline of Key Discoveries
The development of our understanding of the atom has been a gradual process, with many key discoveries contributing to our current knowledge. The following table provides a chronological summary of some of the major milestones in the history of atomic theory:
Dalton’s Atomic Theory
- Proposed by John Dalton in 1803, Dalton’s atomic theory was the first comprehensive model of the atom.
- According to Dalton’s theory, all matter is composed of tiny, indivisible particles called atoms.
- Atoms of the same element are identical in size, mass, and other properties.
- Atoms of different elements have different sizes, masses, and other properties.
- Atoms combine in simple whole-number ratios to form compounds.
Thomson’s Electron Discovery
- In 1897, J.J. Thomson discovered the electron, a negatively charged particle found within atoms.
- Thomson’s experiments with cathode rays showed that electrons are much smaller than atoms and have a negative charge.
- Thomson’s discovery led to the “plum pudding” model of the atom, which proposed that electrons are embedded in a positively charged sphere.
Rutherford’s Nuclear Model
- In 1911, Ernest Rutherford conducted the gold foil experiment, which led to the discovery of the atomic nucleus.
- Rutherford’s experiment showed that most of the atom’s mass is concentrated in a small, positively charged nucleus.
- The nucleus is surrounded by a cloud of negatively charged electrons.
- Rutherford’s model of the atom was a major breakthrough in our understanding of atomic structure.
Bohr’s Atomic Model
- In 1913, Niels Bohr proposed a model of the atom that explained the behavior of electrons in atoms.
- Bohr’s model proposed that electrons orbit the nucleus in specific energy levels.
- Electrons can move from one energy level to another by absorbing or emitting photons of light.
- Bohr’s model was a significant advance in our understanding of atomic structure and laid the foundation for quantum mechanics.
Contributions of Key Scientists
The understanding of the atom has evolved over centuries, with numerous scientists contributing to our current knowledge. From ancient philosophers to modern physicists, their experiments and theories have shaped our understanding of the fundamental building blocks of matter.
Democritus and Aristotle
In ancient Greece, Democritus proposed the idea of atoms as indivisible particles of matter. However, Aristotle’s theory of continuous matter dominated scientific thought for centuries, eclipsing Democritus’ atomic theory.
John Dalton
In the early 19th century, John Dalton revived the atomic theory, proposing that all matter is composed of tiny, indivisible atoms of different weights. His experiments on gases led to the law of partial pressures and the law of multiple proportions, providing evidence for the atomic nature of matter.
J.J. Thomson
In 1897, J.J. Thomson discovered the electron through his experiments with cathode rays. This discovery challenged Dalton’s idea of atoms as indivisible and suggested that atoms have a subatomic structure.
Ernest Rutherford
In 1911, Ernest Rutherford conducted the gold foil experiment, which led to the discovery of the atomic nucleus. He proposed the Rutherford model of the atom, which depicted the atom as a tiny, dense nucleus surrounded by orbiting electrons.
Niels Bohr
In 1913, Niels Bohr proposed the Bohr model of the atom, which introduced the concept of energy levels and explained the emission and absorption of light by atoms. Bohr’s model further refined the understanding of atomic structure and laid the foundation for quantum mechanics.
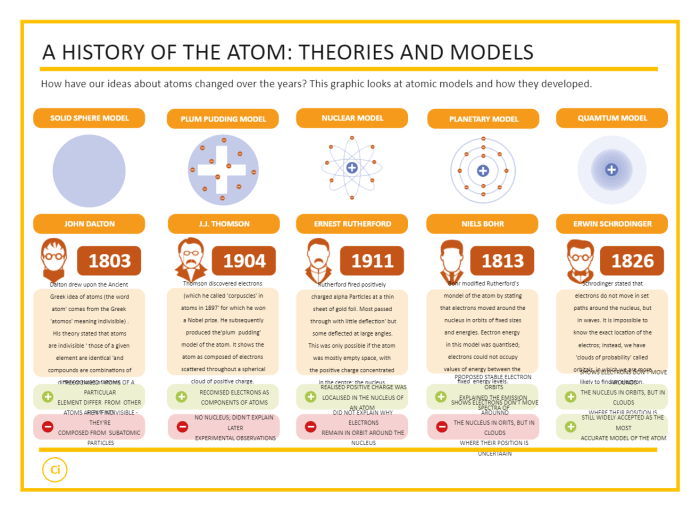
Evolution of Atomic Models
The evolution of atomic models has been a continuous process, with each new model providing a more accurate representation of the atom’s structure. From the ancient Greek philosophers to modern quantum mechanics, scientists have made significant contributions to our understanding of the atom.
The first atomic model was proposed by Democritus in the 5th century BC. He suggested that all matter is composed of tiny, indivisible particles called atoms. However, this model did not provide any details about the structure of atoms.
Plum Pudding Model
In the early 20th century, J.J. Thomson proposed the plum pudding model of the atom. This model suggested that the atom is a sphere of positive charge with electrons embedded in it like plums in a pudding. However, this model did not explain how the electrons were held in place.
Nuclear Model
In 1911, Ernest Rutherford proposed the nuclear model of the atom. This model suggested that the atom has a small, dense nucleus at its center, with electrons orbiting around it. This model was based on Rutherford’s experiments on the scattering of alpha particles by gold atoms.
Quantum Mechanical Model
In the 1920s, Erwin Schrödinger proposed the quantum mechanical model of the atom. This model uses wave mechanics to describe the behavior of electrons in atoms. The quantum mechanical model is the most accurate model of the atom to date.
Impact on Modern Science and Technology

The understanding of the atom has revolutionized various scientific disciplines and technological advancements. Its practical applications span fields such as chemistry, physics, and materials science.
In chemistry, atomic theory has enabled the understanding of chemical bonding, molecular structures, and the periodic table. This knowledge has led to the development of new materials, drugs, and chemical processes.
Nuclear Energy
The discovery of nuclear fission and fusion has unlocked the potential for harnessing atomic energy. Nuclear power plants generate electricity by controlled nuclear reactions, providing a clean and efficient energy source.
Electronics
The understanding of atomic properties has enabled the development of transistors, integrated circuits, and other electronic devices. These advancements have fueled the growth of the electronics industry and led to the miniaturization and increased efficiency of electronic devices.
Medicine
Atomic theory has played a crucial role in medical advancements. Radioisotopes are used in medical imaging and cancer treatment. The development of nuclear medicine has enabled the diagnosis and treatment of various diseases.
Unresolved Questions and Future Directions
Our understanding of the atom is still incomplete, and many questions remain unanswered. One of the most fundamental questions is the nature of dark matter and dark energy, which make up over 95% of the universe but are not yet fully understood.
Another major area of research is the study of quantum mechanics, which governs the behavior of atoms and subatomic particles. Quantum mechanics is a complex and challenging theory, and there are still many unanswered questions about how it works.
Future Directions, History of the atom timeline project
Ongoing research in atomic physics is focused on several key areas, including:
- The development of new experimental techniques to study atoms and subatomic particles.
- The search for new particles and forces.
- The development of new theories to explain the behavior of atoms and subatomic particles.
These research efforts are likely to lead to new discoveries that will deepen our understanding of the atom and its role in the universe.
Question Bank: History Of The Atom Timeline Project
What is the significance of understanding the history of the atom?
Understanding the history of the atom provides insights into the evolution of scientific thought and the development of our current understanding of matter. It allows us to appreciate the contributions of scientists throughout history and trace the progress of scientific discovery.
How has the understanding of the atom impacted modern science and technology?
The understanding of the atom has revolutionized fields such as chemistry, physics, and materials science. It has led to advancements in nuclear energy, electronics, and medicine, among other areas.
What are some unresolved questions in atomic physics?
Ongoing research in atomic physics explores areas such as the behavior of atoms in extreme conditions, the nature of dark matter, and the development of quantum computers.