Embark on an enthralling journey into the realm of chemistry with the Ionic and Covalent Bonds Labster. This interactive simulation unlocks the mysteries of chemical bonding, revealing the fundamental principles that govern the formation and properties of molecules.
Delve into the captivating world of ionic and covalent bonds, unraveling their distinct characteristics and exploring their widespread applications in various industries. Prepare to be captivated as we unveil the profound impact of these bonds on the development of groundbreaking technologies.
Ionic and Covalent Bonds
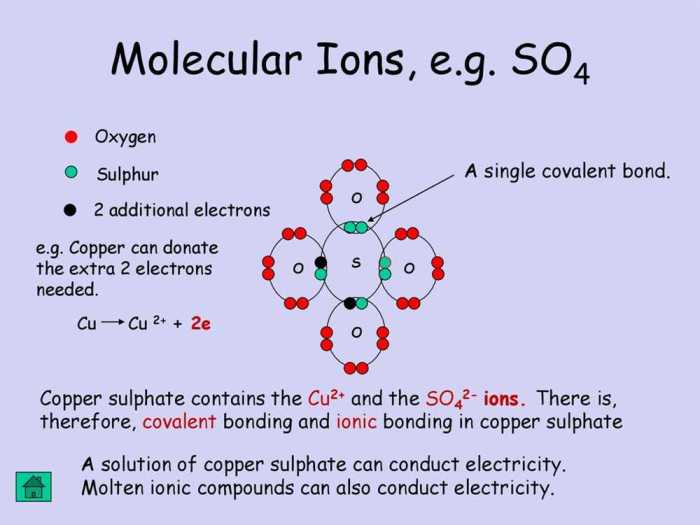
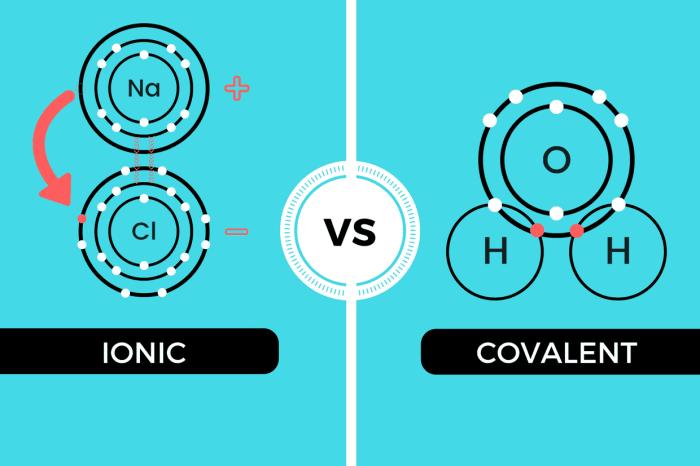
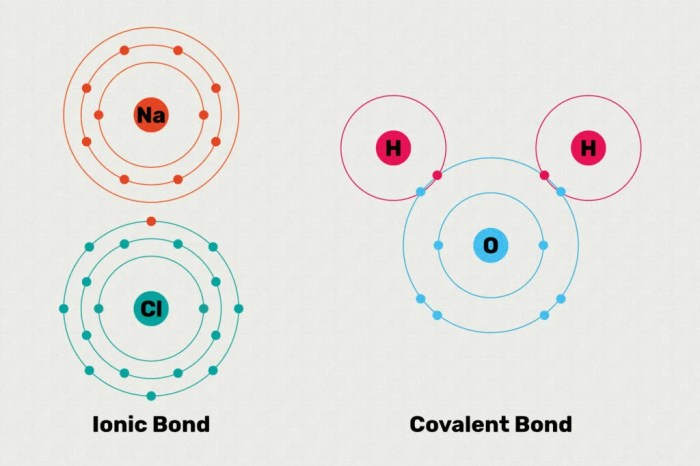
Ionic and covalent bonds are the two main types of chemical bonds. They differ in the way that the atoms are held together. In an ionic bond, one atom transfers one or more electrons to another atom. This creates a positive ion and a negative ion, which are attracted to each other by the electrostatic force.
In a covalent bond, the atoms share one or more pairs of electrons. This creates a covalent bond, which is a strong bond that holds the atoms together.
Properties of Ionic and Covalent Compounds
Ionic compounds are typically hard, brittle, and have high melting and boiling points. They are also good conductors of electricity. Covalent compounds are typically soft, ductile, and have low melting and boiling points. They are also poor conductors of electricity.
Labster Simulation
The Labster simulation on ionic and covalent bonds is an interactive virtual environment that allows students to explore the properties of these two types of chemical bonds.
The simulation begins with a brief introduction to ionic and covalent bonds. Students then use the simulation to create their own ionic and covalent compounds and investigate their properties. They can measure the bond lengths, bond strengths, and melting points of their compounds and compare them to the properties of real-world compounds.
Benefits of Using the Simulation
The Labster simulation on ionic and covalent bonds has several benefits for teaching and learning about these two types of chemical bonds.
- Interactive and engaging:The simulation allows students to interact with the material in a hands-on way, which makes the learning process more engaging and enjoyable.
- Visual and intuitive:The simulation uses 3D graphics to represent the bonds between atoms, which makes it easy for students to visualize the concepts being taught.
- Customizable:The simulation allows students to create their own compounds and investigate their properties, which gives them a deeper understanding of the factors that affect bond strength and stability.
Applications of Ionic and Covalent Bonds
Ionic and covalent bonds play a crucial role in numerous aspects of everyday life and technological advancements.
Ionic bonds, characterized by the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions), are essential in the formation of salts, which are widely used in various industries.
Applications in Everyday Life
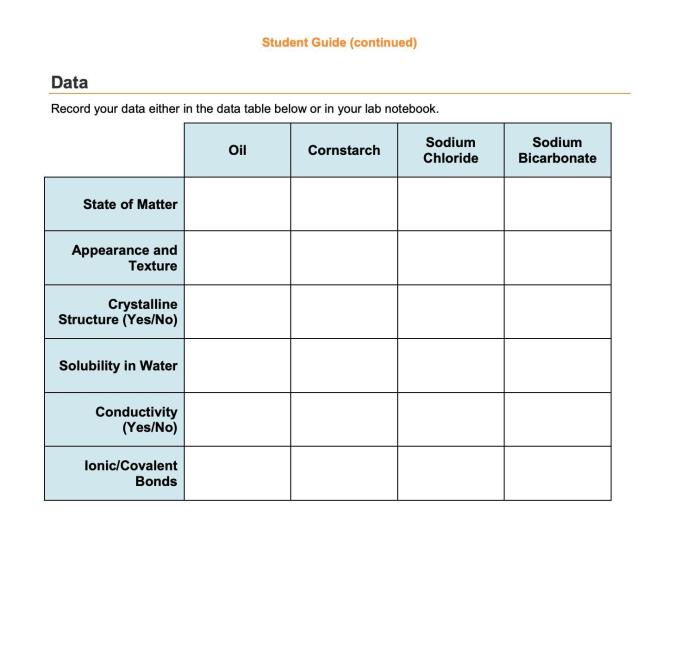
- Table salt (sodium chloride) is a common ionic compound used as a seasoning and preservative.
- Baking soda (sodium bicarbonate) is an ionic compound used as a leavening agent in baking.
- Toothpaste contains ionic compounds like fluoride, which helps strengthen teeth and prevent cavities.
Applications in Industries
- Electroplating: Ionic compounds are used to coat metals with other metals, improving their properties such as corrosion resistance and electrical conductivity.
- Batteries: Ionic compounds are essential components of batteries, where they facilitate the flow of ions between electrodes.
- Water purification: Ion exchange resins are used to remove impurities from water by exchanging ions.
Covalent bonds, involving the sharing of electron pairs between atoms, are equally important in various fields.
Applications in Biotechnology, Ionic and covalent bonds labster
- DNA and RNA: The genetic material of living organisms is composed of covalent bonds between nucleotides.
- Proteins: Covalent bonds hold together the amino acids that make up proteins, which are essential for biological functions.
- Enzymes: Covalent bonds are crucial for the structure and function of enzymes, which catalyze biochemical reactions.
Applications in Materials Science
- Polymers: Covalent bonds form the backbone of polymers, which are used in a wide range of materials, from plastics to fibers.
- Graphene: Covalent bonds between carbon atoms give graphene its unique properties, making it a promising material for electronics and other applications.
- Nanomaterials: Covalent bonds play a role in the synthesis and properties of nanomaterials, which have potential applications in medicine, energy, and electronics.
Advanced Concepts
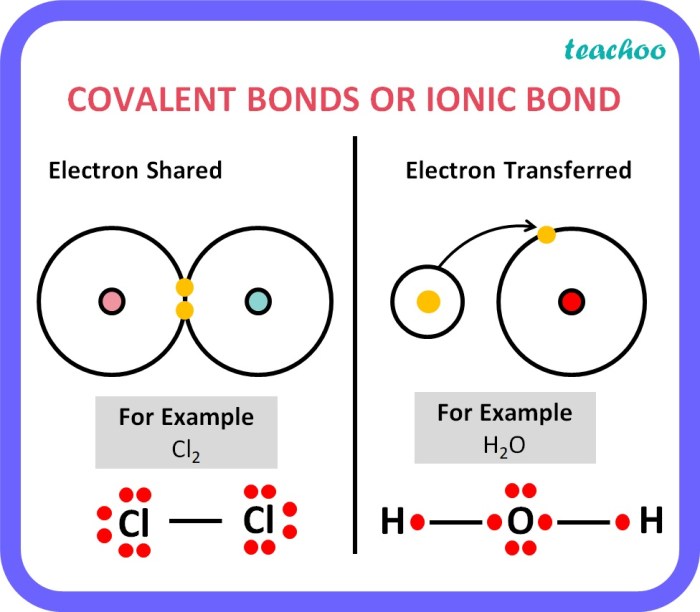
Beyond the basic principles of ionic and covalent bonding, several advanced concepts play crucial roles in understanding the nature of chemical bonds and their impact on molecular properties.
Electronegativity
Electronegativity refers to the ability of an atom to attract electrons towards itself. It is a fundamental property of elements that determines the type of bond formed when atoms interact. A higher electronegativity value indicates a stronger attraction for electrons.
When atoms with significantly different electronegativities interact, they form ionic bonds. Conversely, when atoms have similar electronegativities, they tend to form covalent bonds.
Resonance
Resonance is a concept that describes the delocalization of electrons in certain molecules. It occurs when multiple valid Lewis structures can be drawn for a molecule, each with different arrangements of double and single bonds. These structures are called resonance structures.
Resonance stabilizes the molecule by distributing the electrons over several atoms, resulting in a lower overall energy state.
Quantum Mechanics
Quantum mechanics provides a rigorous mathematical framework for understanding the behavior of electrons and their interactions within molecules. It introduces concepts such as wave functions and orbitals, which describe the probability of finding electrons in specific regions of space. Quantum mechanics enables the calculation of bond lengths, bond strengths, and other molecular properties with remarkable accuracy.
Clarifying Questions: Ionic And Covalent Bonds Labster
What is the fundamental difference between ionic and covalent bonds?
Ionic bonds arise from the electrostatic attraction between oppositely charged ions, while covalent bonds result from the sharing of electrons between atoms.
Can you provide an example of an ionic compound?
Sodium chloride (NaCl) is a classic example of an ionic compound, formed by the transfer of an electron from sodium to chlorine.
What are the key properties of covalent compounds?
Covalent compounds typically exhibit lower melting and boiling points compared to ionic compounds, and they are generally poor conductors of electricity.